-
Prospective Students
-
About the GSBS
- About the GSBS
-
Quick Facts
- Administered by MD Anderson and UTHealth Houston
- Located in The Texas Medical Center (TMC), the world's largest medical center
- Both MD Anderson and UTHealth Houston are accredited by the Southern Association of Colleges and Schools Commission on Colleges (SACSCOC)
- Over 600 faculty members with expertise in the latest biomedical research
-
- MS PROGRAMS
- PHD PROGRAMS
-
MD/PhD PROGRAM
- MD/PhD Program
- Participating Institutions/Entities
-
Student Research Day 2020
-
Admissions
- Admissions
-
Admission FAQs
- What factors are considered in admissions decisions?
- What is the minimum GPA required to apply?
- Do you require interviews?
- When will I be notified regarding interviews?
- What are the application deadlines?
- What if my recommender does not have an institutional letterhead or an institutional email address?
- How can I get an assistantship?
- How can I get an assistantship if I am seeking a MS degree?
-
Admissions Office
6767 Bertner Avenue
S3.8344 Mitchell BSRB
Houston TX 77030
-
Research
- Research
- Research Interests
-
Student Research Day 2020
- Student Life
-
About the GSBS
- Current Students
- Faculty
- Alumni
- Academics
- Give
- Events
- Map
- Contact Us
-
About the GSBS
About the GSBS
Quick Facts
- Administered by MD Anderson and UTHealth Houston
- Located in The Texas Medical Center (TMC), the world's largest medical center
- Both MD Anderson and UTHealth Houston are accredited by the Southern Association of Colleges and Schools Commission on Colleges (SACSCOC)
- Over 600 faculty members with expertise in the latest biomedical research
-
MS PROGRAMS
Individualized MS Program in Biomedical Sciences
-
PHD PROGRAMS
PhD Programs
-
MD/PhD PROGRAM
MD/PhD Program
Participating Institutions/Entities
-
Admissions
Admissions
Admission FAQs
- What factors are considered in admissions decisions?
- What is the minimum GPA required to apply?
- Do you require interviews?
- When will I be notified regarding interviews?
- What are the application deadlines?
- What if my recommender does not have an institutional letterhead or an institutional email address?
- How can I get an assistantship?
- How can I get an assistantship if I am seeking a MS degree?
Admissions Office
6767 Bertner Avenue
S3.8344 Mitchell BSRB
Houston TX 77030 -
Research
Research
Research Interests
Student Research Day 2020
-
Student Life
Student Life
Student Organizations
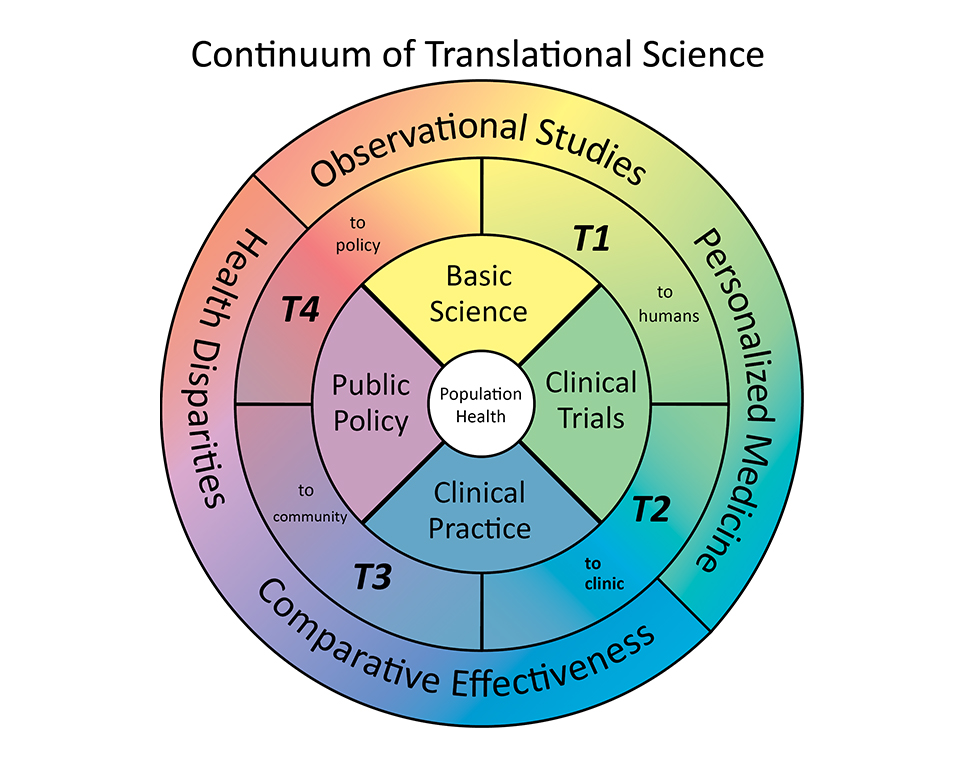
In general, "translational" science can be classified into four categories: T1, T2, T3, T4.
T1 / T2 [translating to patients]: making & adapting laboratory discoveries for treatment of human disease
T3 / T4 [translating to community]: establishing, disseminating, & implementing best practices for clinic- and community-based interventions
Translational research is not unidirectional; it is a type of continuum. Community observations can inspire laboratory experiments just as laboratory animal studies can influence clinical treatment and community prevention initiatives. And, at every T level, information needs to be disseminated for implementation.
In this program:
Clinical Research involves direct human subject interaction.
Translational Research can:
- improve human health by reducing incidence, morbidity, or mortality of disease
- make discoveries in a research laboratory and/or preclinical study, which can be developed into human studies
- enhance the adoption of best practices in the community; comparative effectiveness of prevention and treatment
[definitions modified from recommendations by the NCI Translational Working Group (2006) and the CTSA RFA-RM-10-020]
CCTS T32 Overview
-
About CCTS T32
The NIH Clinical and Translational Science Award (CTSA) program was launched in 2006 and is funded by the National Center for Advancing Translational Science (NCATS). The CTSA program seeks to create “...a definable home for clinical and translational research.” To date, there are more than 50 CTSA Program hubs at institutions across the country.
UTHealth Houston was one of the first universities, of only twelve, granted a CTSA in 2006, and the UTHealth Houston award was renewed in 2012 and 2019. The UTHealth Houston CTSA is held in partnership with MD Anderson Cancer Center and Memorial Hermann Hospital, and the program extends across all schools offering doctoral training in the UTHealth Houston system.
UTHealth Houston Schools:
MD Anderson UTHealth Houston Graduate School
McGovern Medical School
Cizik School of Nursing
McWilliams School of Biomedical Informatics
School of Dentistry
School of Public HealthSchool of Behavioral Health Sciences
The UT Center for Clinical and Translational Sciences (CCTS) serves as the central organizing entity for all of the Houston CTSA components, which includes T32 and KL2 training programs. The CCTS T32 Program is coordinated through the MD Anderson UTHealth Houston Graduate School, which is a joint venture of UTHealth Houston and MD Anderson Cancer Center. The T32 program is open to PhD (including MD/PhD) predoctoral students from across the UTHealth Houston Schools and postdoctoral trainees at UTHealth Houston and MD Anderson Cancer Center who meet the eligibility criteria (see Trainee Eligibility). Trainees accepted into the T32 Program receive a stipend, health insurance, and modest travel funds in addition to resources and training in clinical and translational science.
Because UTHealth Houston is comprised of schools, trainees’ research projects can be incredibly diverse – a characteristic that the T32 Program views as a distinct advantage. Appointment to the T32 program requires trainees to conduct scientifically rigorous research studies that are either clinical or translational in nature. The studies may fall anywhere on the translational research continuum and may be classified into any field, from epidemiological health disparities practices to pre-clinical cancer or cardiovascular therapies to structural and functional imaging of disease states.
Further, through the Texas Regional CTSA Consortium, the CCTS T32 Trainees have access to resources and training opportunities at the four other CTSA institutions in Texas:
UT Southwestern Medical Center CTSA Program (Dallas)
UT Health San Antonio Institute for Integration of Medicine & Science
The University of Texas Medical Branch Institute for Translational Sciences (Galveston)
-
Leadership
Co-Directors
Joya Chandra, PhD
Professor
Department of Pediatrics Research and Department of Epigenetics and Molecular Carcinogenesis
Co-Director, Center for Energy Balance in Cancer Prevention and Survivorship
MD Anderson Cancer CenterJeffery A. Frost, PhD
Associate Dean for Academic and Faculty Affairs, MD Anderson UTHealth Houston Graduate School of Biomedical Sciences
Professor, Integrative Biology & Pharmacology
Co-InvestigatorsShervin Assassi, MD, MS
Professor, Division Director, Division Of Rheumatology
McGovern Medical School at UTHealthRobert C. Bast, MD
Vice President for Translational Research
Internist and Professor of Medicine, Department of Experimental Therapeutics
Harry Carothers Wiess Distinguished University Chair for Cancer Research
MD Anderson Cancer Center -
Training Curriculum
The T32 is an enrichment pathway for highly qualified predoctoral students and postdoctoral fellows. It prepares them to rapidly integrate their skills into the clinical and translational sciences workforce.
We accomplish this by targeting stage-specific (pre- versus post-doctoral) training that comprises a competency-based curriculum along with career development enrichment pertinent to the translational workforce.
Core Competencies in Translational Research
Our competency-based curriculum encompasses fundamental, thematic areas of clinical and translational sciences but also allows considerable flexibility in how each competency is achieved to fit each trainee’s research interests and career stage-specific goals.
Clinical & Community Engagement
All T32 trainees are required to have a clinical mentor and obtain compliance and other training to undertake shadowing every month. This opportunity not only allows exposure to a clinical setting but also community (patient) engagement to increase their understanding of the population most immediately affected by their research (T1/T2 translation) and to promote understanding and awareness of the public population that will benefit from translational research (T3/T4).
Translational Science Seminar Series
The T32 Core Monthly Seminar Series promotes peer and near-peer education, discussion, and debate about translational science and enhances research communication skills and career development.
Training Plan Agreement
Upon acceptance into the T32 Program, Trainees are expected to confer with their mentors to devise a customized Training Plan outlining proposed coursework and clinical shadowing. Once approved by the T32 Co-Directors, the Training Plan is signed by the Trainee, Mentor, and Co-Directors. For competitive renewal of the T32, Trainees must show regular participation in T32 Program activities, progress along their T32 Training Plan, and progress within their home degree program.
National T32 Predoctoral Meeting
All trainees are required to attend and present their work at the annual meeting of the Association for Clinical and Translational Science at least once during their T32 training period. At the meeting, T32 programs from across the country convene with opportunities to gain advocacy experience by visiting Congress on Capitol Hill and to network with translational scientists from around the country.
Questions about the T32 Training Curriculum should be addressed to Jewel Elliott (713-500-9886).
-
Trainee Eligibility
Research Eligibility Criteria
To be eligible for the T32 Program, an applicant's research must be broadly categorized as clinical or translational science.
- Clinical Research involves direct human subject interaction.
- Translational Research may:
- improve human health by reducing incidence, morbidity, or mortality of disease
- make discoveries in the research laboratory and/or preclinical study, which are readily developed into studies in humans
- enhance the adoption of best practices in the community; comparative effectiveness of prevention and treatment
Trainee Eligibility Criteria
The CCTS T32 Program supports a total of 6 predoctoral and 3 postdoctoral trainee slots that provide up to two (2) years of full stipends, tuition, health insurance, and modest travel funds.
The T32 Program is open to:
PhD Students:
2nd or 3rd year
Full-time, good academic standing at one of the seven UTHealth Houston Schools:
Cizik School of Nursing
McGovern Medical School
MD Anderson UTHealth Graduate School
McWilliams School of Biomedical Informatics
School of Dentistry
School of Public Health
School of Behavioral Health SciencesFull time, good academic standing at the following partner organizations:
The University of Texas Rio Grande Valley
Rice University
UT Tyler Health Science CenterMD/PhD Students:
1st or 2nd year of the PhD portion of training
Full-time, good academic standing at one of the following:
MD Anderson UTHealth Graduate School
McGovern Medical SchoolPostdoctoral Scholars:
PhD degree with 0-2 years of postdoctoral training at any institution.
Postdoctoral appointment at one of the following institutions:
UTHealth Houston Cizik School of Nursing
UTHealth Houston McGovern Medical School
UTHealth Houston MD Anderson Graduate School
UTHealth Houston McWilliams School of Biomedical Informatics
UTHealth Houston School of Dentistry
UTHealth Houston School of Public Health
UTHealth Houston School of Behavioral Health Sciences
The University of Texas Rio Grande Valley
Texas Tech Health - El Paso
Rice University
UT Tyler Health Science Center* All applicants must be a U.S. citizen or permanent resident.*
Awardees are expected to devote 100% professional effort to this award; no other grant salary support or outside employment is permitted.
Please note: No trainee may have a cumulative total of more than 5 years of NRSA support, including other training grant (T32) appointments and individual Fellowships (i.e. F30, F31, F32)
NIH regulations do not permit the provision of stipends for students pursuing a professional degree or for students in dual-degree programs (e.g., MD/PhD) while they are enrolled in the professional school portion of their training.
-
Applications
The 2026 Application period is currently closed.
KEY DATES:
August 31, 2025 Applications Due Early September Application Review Early October Candidate Selection January 1, 2026 Appointments Begin Applying to the T32 Program
Appointments to the training grant are for 12 months with the option of competitive renewal for an additional 12-month appointment for a maximum of 2 years. Renewal of appointment is contingent upon annual evaluation of performance metrics and full participation in the program.
All T32 Trainees are expected to develop a curriculum/training plan that will enable them to achieve core competencies in clinical and translational research. These competencies are fulfilled by conducting research, clinical shadowing, completing clinical and translational courses offered by participating CCTS schools and institutions, and participating in seminars, research retreats, and other activities that provide training in clinical and translational research.
Eligibility Criteria
To be eligible for the T32 Program, applicants must be predoctoral student or postdoctoral trainee currently conducting a clinical or translational research project. Additional eligibility criteria can be found above.
Application Materials
Applications are to be submitted online: T32 Application Portal. All supporting documents should be uploaded as individual PDF files. Text is single spaced, 11pt Arial font, with ½-inch margins on all sides.
- Cover Form (download here)
- Trainee Biosketch (5 pages)
- Please include an NIH-formatted Fellowship Applicant Biosketch
- NIH Biosketch Instructions along with examples can be found at: https://grants.nih.gov/grants/forms/biosketch.htm
- Mentor Biosketch (5 pages)
- Please include an NIH-formatted Biosketch
- Co-Mentor Biosketch (if applicable) (5 pages)
- Please include an NIH formatted Biosketch
- Clinical Mentor Biosketch (5 pages)
- Please include an NIH-formatted Biosketch
- Career Goals and Training Plan (2 pages)
- Describe your long-term career goals and how the combination of past research experiences and the T32 training program will contribute to achieving these goals
- Summarize the scientific and professional development activities you will undertake, including plans for clinical shadowing/activities, and explain how these activities will facilitate the transition to each subsequent career stage
- Research Proposal
- Specific Aims (1 page)
- A summary of the proposed project (rationale/background, gap in knowledge), hypothesis statement, and 2-3 specific aims that briefly outline how each aim will address the hypothesis
- The clinical and/or translational significance to human health must be clearly outlined
- Research Strategy (5 pages)
- Research proposal detailing the research project, specific aims, experimental approaches, anticipated outcomes, potential pitfalls and alternative approaches
- Proposals will be evaluated for
- Clinical and/or translational significance to human health
- Approach
- Innovation
- Literature Cited (no page limit)
- Specific Aims (1 page)
- Letters of Support (2 pages each)
- Please provide letters of support from your Mentor, Co-mentor (if applicable), and clinical mentor who you plan to shadow
- Letters should demonstrate the candidate’s commitment to a career in clinical/translational sciences and how the trainee will benefit from training in the T32 program
- Letters of Support need to include an official letterhead and formal signature
Review Criteria
Applications are reviewed by the T32 Leadership and Internal Advisory Board. Appointments are made based on the scientific merit of the proposal including: the student's accomplishments; the mentor's accomplishments and ability to support the student's clinical/translational research; and the presence or potential for clinical and basic science collaborations.
For more information on trainee eligibility or application, please contact Jewel Elliott (713.500.9886).
CCTS T32 Policies
-
Publication Policy
Grant Acknowledgement
Each publication, press release, or other document (e.g., posters) referencing research supported by an NIH award (including the CCTS T32) must include an acknowledgment of NIH award support and a disclaimer such as:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers T32TR004905, T32TR004904 and UM1TR004906. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Public Access
(NOT-OD-08-033)
Award recipients are required to comply with the NIH Public Access Policy, such that scientists are required to submit final peer-reviewed journal manuscripts that arise from NIH funds to the digital archive PubMed Central (PMC), upon acceptance for publication. To help advance science and improve human health, the Policy requires that these papers are accessible to the public on PMC no later than 12 months after publication.
The appropriate method for submitting your manuscript is determined by your journal’s publisher. For specifics on how to submit your article to PMC, see publicaccess.nih.gov/submit_process.htm.
For additional information, please visit: publicaccess.nih.gov
For T32 Annual Report
Upon publication, please also send the PMID and/or PMCID number for inclusion in annual reporting.
T32 Trainees are required to have an ORCID number as mandated by NIH notice NOT-OD-19-109 and to facilitate the T32 Program maintaining a publication list for annual reports.
-
Travel Policy
To Scientific Meetings
There are modest travel funds available to CCTS T32 Trainees. Travel fund requests must be made at least two (2) months in advance of proposed travel and must include:
- Meeting website
- First-author abstract to be presented by the Trainee at the meeting
- Letter of support from the Mentor, outlining the educational relevance of the proposed travel
For all travel, Trainees are asked to seek travel funds from their School, Program, or Department in addition to T32 travel funds.
T32 National Meeting
Trainees are encouraged to attend the National T32 Predoctoral Meeting as it is an opportunity to network with diverse translational scientists around the country.
Questions regarding T32 Travel should be directed to Jewel Elliott (713-500-9886).
Contact Us
Co-Directors
Joya Chandra, PhD
[email protected]
Jeffrey A. Frost, PhD
[email protected]
Co-Investigators
Shervin Assassi, MD
[email protected]
Robert C. Bast, MD
[email protected]
Program Manager
Jewel Elliott, MPA
[email protected]







